Companies developing new products face many barriers to market entry, including budget, timelines, and pressure to be innovative. Often, the greatest challenge is figuring out what you want to say about your product—and what you can say—based on what it contains, how it works, and where you want to sell it.
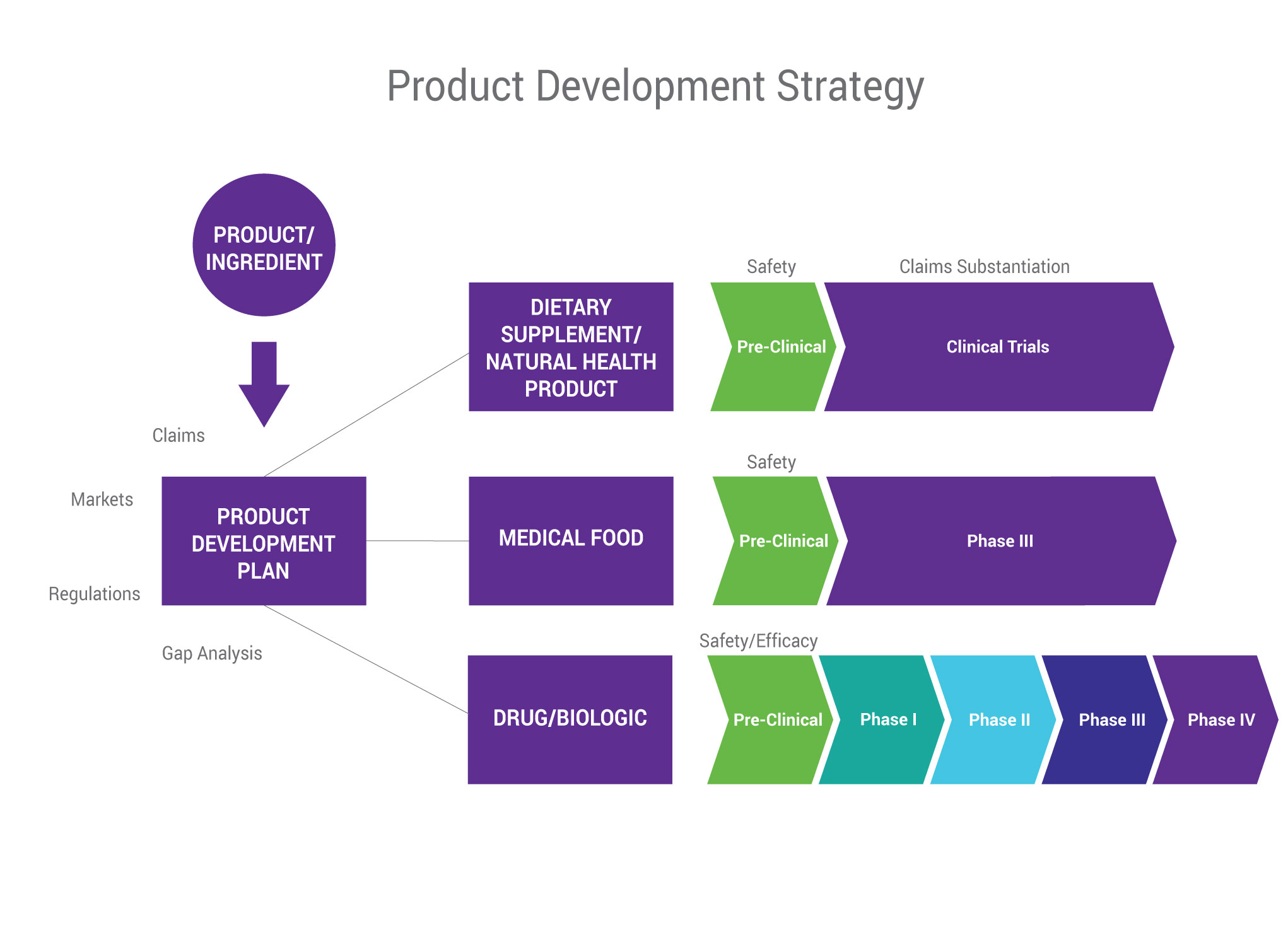
A strategic product development plan that focuses on the end goal—the label claim—helps ensure a timely, more efficient launch while expediting the R&D process, minimizing cost of failure, and delivering a more targeted development plan overall.
At SGS Nutrasource, we take a “pharmaceutical-lite” approach to dietary supplement development to help our clients successfully gain market access. Adopting key aspects from the pharmaceutical industry, without the need for a pharma-sized R&D budget, provides our clients with opportunities for prolonged market access, additional claims, further regulatory classifications, and future development opportunities.
We will work with your team to build a focused product development plan that includes an assessment of the risks and benefits of potential regulatory routes, full scientific literature reviews, non-clinical testing and analytical testing summaries, and possible health and marketing claims. Our product development plans also include a comprehensive pre-clinical and/or clinical roadmap to achieve market success in your desired jurisdictions.
Learn how we can help you develop a strategic product development plan tailored to your needs and objectives.
 Skip to main content
Skip to main content


